Sep 03,2024
By: BPC
1 The Influence of Cell Temperature on the Operation of Electrolyzers
1.1 The influence of electrolyzer cell temperature on Cell voltage
The influence of electrolyzer cell temperature on cell voltage is mainly reflected in the following three aspects: metal conductor resistance, electrolyte resistance, and ion membrane resistance. For metal conductors, as the temperature increases, the vibration of metal cations inside the conductor intensifies, hindering the movement of electrons and resulting in an increase in resistivity; For electrolytes, as the temperature increases, the movement speed of freely moving ions in the solution accelerates, resulting in a decrease in electrical resistivity; For ion exchange membranes, as the temperature increases, the membrane expands, the pores increase, and the membrane resistance decreases. Based on the above analysis, the impact of increasing slot temperature on the decrease of ohmic voltage in metal conductors, electrolyte, and ion membrane is not the same pattern. But according to actual operating experience, as the tank temperature increases, the tank voltage decreases.
When the current density, temperature, and alkali concentration of different electrolytic cells are different, it is impossible to compare the voltage operation of the cells. Therefore, the formula for correcting the cell voltage is derived as follows:
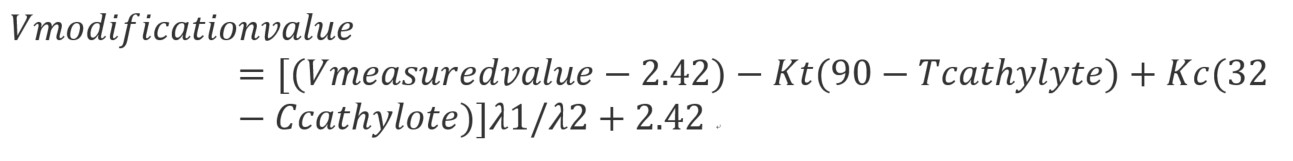
The Vmodification value in the formula is the corrected average cell voltage, V; Kt is the temperature correction coefficient, 0.013/℃; KC is the concentration correction factor, 0.017/%;λ1is the corrected operating current density, KA/m2; λ2 is the actual operating current density, KA/m2.
The Kt in the formula is the temperature correction coefficient. Making a further analysis of this coefficient reveals the relationship between temperature and process power consumption, where for every one degree Celsius increase in temperature, the DC process power consumption decreases by approximately 13 kW·h.
1.2 The influence of electrolyzer temperature on current efficiency
When the electrolyzer temperature is below 90℃, as the electrolyzer temperature increases, the pore size of the ion membrane increases, and the migration of sodium ions in the membrane increases, resulting in an increase in current efficiency. However, due to the increase in pores, it will also lead to an increase in the salt content in the alkali and the production of chlorates to a certain extent.
1.3 The Influence of Electrolyzer Temperature on the Safe Operation of Electrolyzer Cells
For the operation of electrolyzer cells, cell temperature is not only an indicator of economic operation, but also an indicator of safe operation. In the original high current density electrolyzer cell, the temperature control range of the cell is below 90℃, on this basis, the higher the cell temperature is, the lower the voltage of the electrolyzer cell is. After the zero pole distance transformation of the electrolyzer cell is completed, the ion membrane is tightly attached to the cathode of the electrolytic cell, and the control of various indicators of the electrolyzer cell is more strict. The cell temperature is generally required to be controlled below 87℃. If the slot temperature is too high, it may lead to aging of the slot frame gasket, and in severe cases, there is a risk of leakage; On the other hand, when the tank temperature exceeds 90℃, the water to steam ratio inside the unit tank increases, leading to electrolysis of water and the formation of water bubbles on the ion membrane.
2 Control Principle and Process Flow of Electrolytzer cell temperature
The reaction of electrolyzing saturated saline solution is a heat resistant reaction, and the reaction heat is removed by cooling the cathode liquid to maintain the temperature stability of the entire electrolysis system. Taking Inner Chlor-Alkali Plant A as an example, the specific process flow is as follows: the catholyte from 8 electrolytic cells is collected and enters the catholyte circulation tank D-270. It is then pumped by the catholyte circulation pump P-274 to the catholyte high-level tank D-273. Some of the alkali solution in the figure is sent to the circulating alkali solution heat exchanger E-273, and the temperature of the circulating alkali solution is controlled by circulating water heat exchange. 32% of the alkaline ions in the high-level tank D-273 of the cathode liquid flow into the electrolytic cell, and pure water (process condensate) is added along the way to adjust the concentration of the alkaline solution. It can be seen from the process flow of the cathode liquid in the electrolytic cell that the temperature of the 8 electrolytic cells is controlled as a whole through the circulating alkali solution heat exchanger E-273. The process flow diagram of the cathode liquid system in the electrolytic cell is shown in Figure 1.
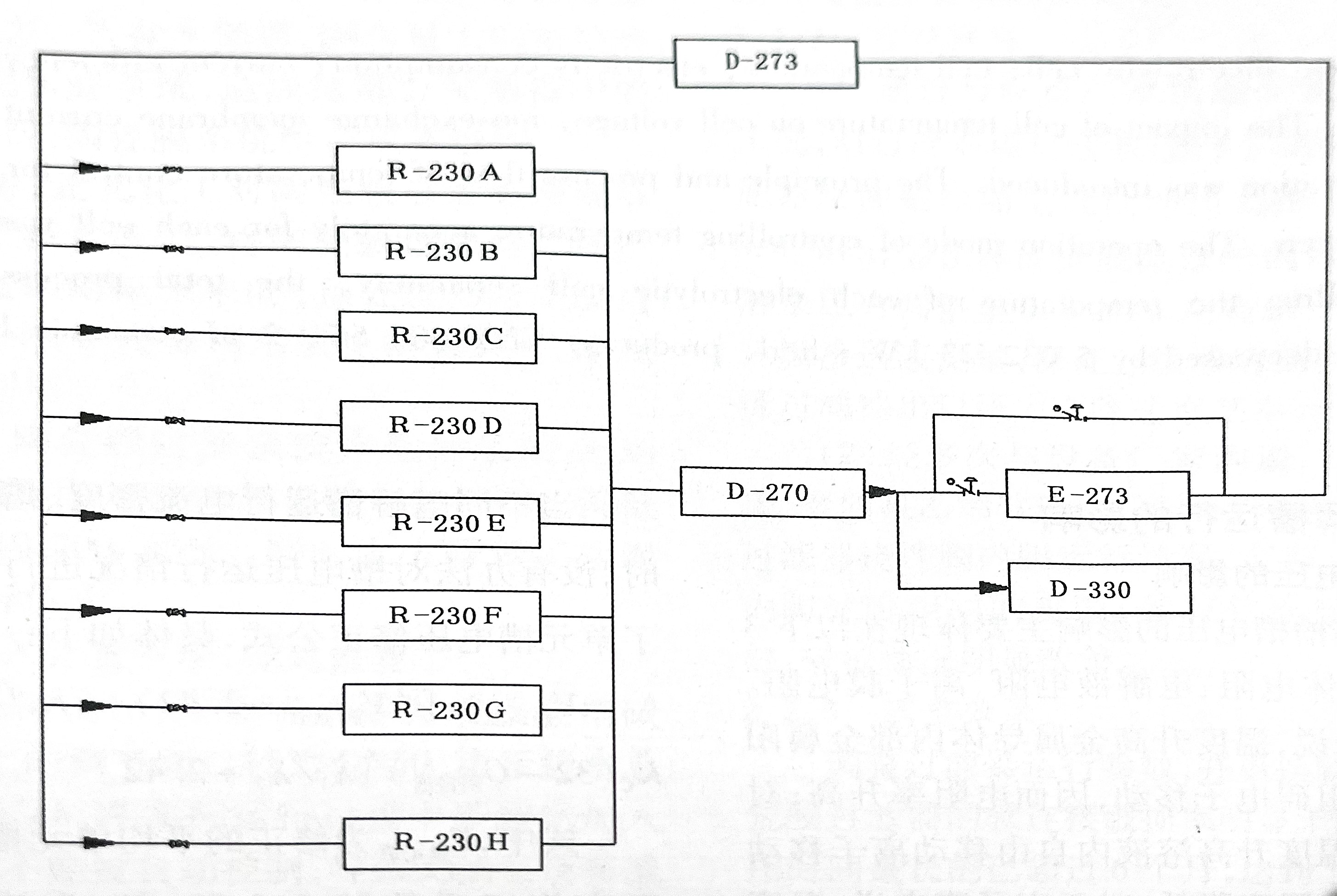
Fig. 1 Process flow of electrolytic cell catholyte system
3 Temperature Control of Electrolytzer Cell
The current update cycle of the ion membrane at Inner Chlor-Alkali Plant A is about 2 years. Due to differences in the usage time of the ion membrane and the condition of the tanks, there are certain deviations in the temperature and voltage of the 8 electrolyzer. The specific operating data is shown in Table 1.

Table 1 Operational data of electrolyzer cells before the project is implemented
From the above statistics, it can be seen that under the same operating current, due to differences in the condition of the cells and the operating cycle of the ion membrane, there is a certain deviation in the temperature of the 8 electrolyzer cells. The temperature of the G and H cells has basically reached or exceeded the control maximum limit, while the temperature of the A, B, C, D, E, and F cells is relatively low, and there is still some room for adjustment. At present, the lowest and highest temperatures of the 8 electrolyzer cells are 83.7℃ and 86.91℃, with a deviation of 3.21℃. This is mainly due to the gradual deposition of impurities in the saltwater on the surface or inside of the membrane as the ion exchange membrane is used for a longer period of time, resulting in a decrease in membrane voltage, an increase in heat generation, and ultimately an increase in tank temperature. In addition, it is also related to the situation of the electrolytic cell.
The temperature control of the electrolyzer cell adopts the centralized control of catholyte circulating alkali solution, which cannot achieve separate adjustment of the electrolyzer cell temperature. In order to ensure safe and stable production, the highest cell temperature among the 8 electrolytic cells can only be used as the benchmark for control. If the inlet alkali solution temperature of each electrolytic cell can be separately controlled, it can further improve the operating temperature of individual electrolyzer cells with low cell temperatures and further reduce process electricity consumption.
4 Process Design of Temperature Control Project for Electrolyzer Cell Partitioning
According to the operation of the electrolyzer cells of Inner Chlor-Alkali A, the maximum cell temperature of C, E, G, and H cells has basically reached the maximum limit. If the temperature of the circulating alkali solution at the outlet of E-273 can be increased, the overall cell temperature of the 8 electrolyzer cells will increase significantly, and the cell temperature of C, E, G, and H cells will exceed 87℃, which cannot meet the safety control requirements. Therefore, it is considered to add plate heat exchangers (E-230, alkali cooler) at the inlet of electrolyzers C, E, G, and H, using cooling water to cool down and reduce the temperature of the alkali solution entering the tank, thereby lowering the temperature of the alkali solution exiting the tank. This can increase the overall temperature of other electrolyzers and achieve the goal of controlling the temperature of the electrolyzer separately. The process design flowchart of the temperature control project for electrolyzer cell partitioning is show in Figure 2.
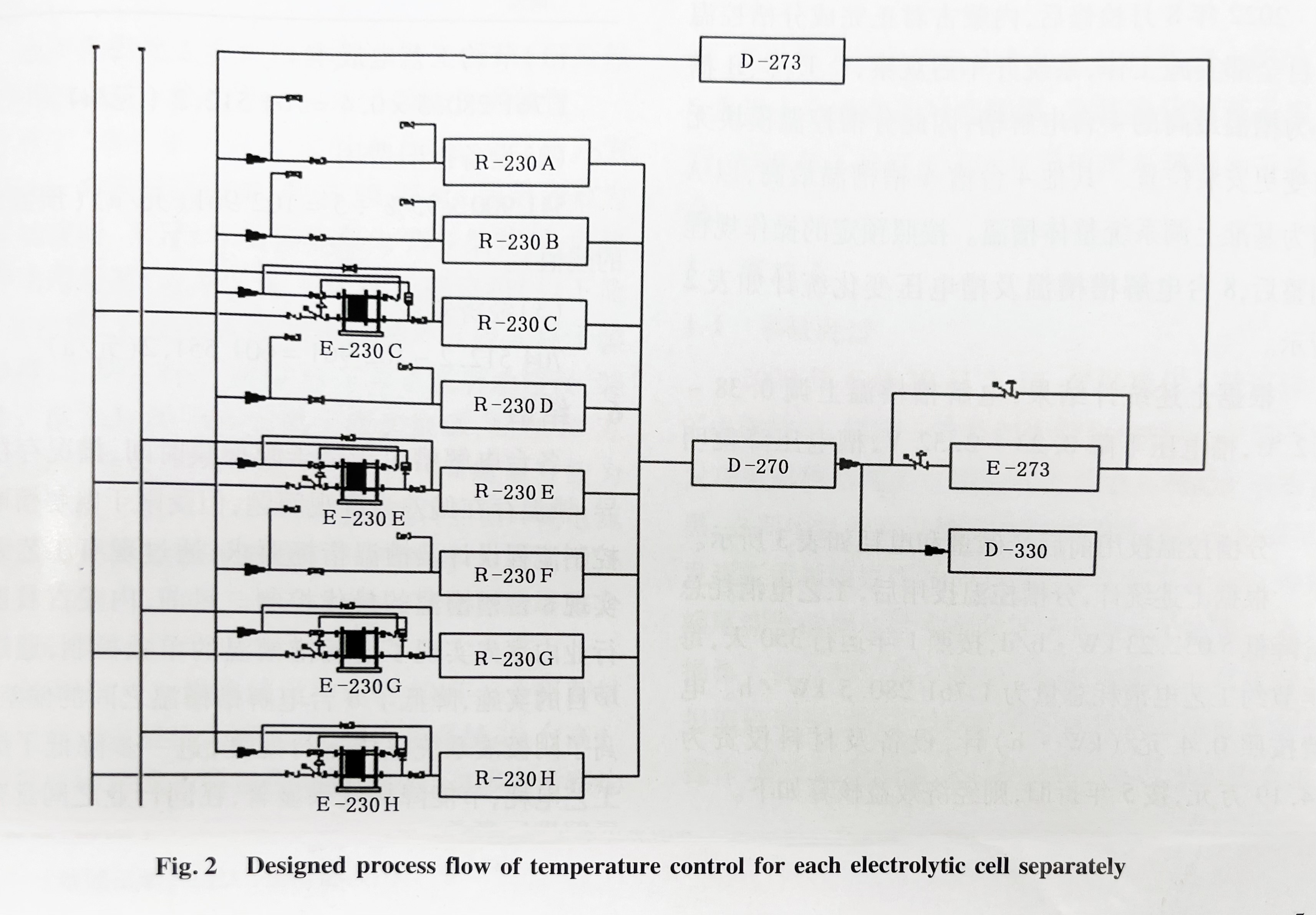
The replacement cycle of ion membranes in the electrolytic cells of Inner Chlor-Alkali Plant A is 2 years, with four electrolyzer cell ion membranes replaced annually. Therefore, the preliminary implementation plan is considered as follows: first, install four segmented temperature controlled plate heat exchangers, and the other four electrolyzer cells will simultaneously complete the installation of circulating water pipelines, alkali liquid pipelines, and remote temperature transmission. The civil foundation of the plate heat exchangers will be made and related cables will be laid. In the later stage, based on the temperature of each electrolytic cell without using segmented temperature controlled plate heat exchangers, the segmented temperature controlled plate heat exchangers, inlet and outlet short sections, and circulating water automatic control valves can be installed as a whole module (segmented temperature control module) in any electrolytic cell according to the temperature of each electrolytic cell.
Expected implementation effect: Taking Inner Chlor-Alkali Plant A as an example, the C, E, G, and H electrolyzers have the highest temperature. Temperature controlled plate heat exchangers are installed at the alkali solution inlet of these 4 electrolyzers to control the tank temperature below 87 degrees Celsius. Except for the four electrolyzer cells mentioned above, cell A has the highest temperature. If the temperature of the alkali solution at the outlet of E-273 is increased to adjust the overall temperature of the electrolyzer cell, assuming that the temperature rise of each cell is the same, then the temperature of cell A is the highest. If the temperature of the alkali solution at the outlet of E-273 is increased to adjust the overall temperature of the electrolyzer cell, assuming that the temperature rise of each cell is the same, the adjustment endpoint is when the temperature of cell A reaches 87℃. At this point, the temperatures of electrolyzers C, E, F, G, H, and D are all around 87℃, and the temperatures of electrolyzers A, B, and D are increased by 1.9℃.
5 Implementation Effect and Benefit Analysis of Temperature Control Project for electrolyzer cell partitioning
After the maintenance in August 2022, Inner Chlor-Alkali Plant A completed all the implementation work of the temperature control project for the electrolyzers separately. After the system was operated, observation showed that the C, E, G, and H electrolyzers were still the four electrolyzers with the highest temperature, so there was no need to change the installation position of the electrolyzers temperature control module. The temperature of electrolyzer A is the highest among the other 4 electrolyzers, and the overall electrolyzers temperature of the system is adjusted based on electrolyzer A. After adjusting according to the predetermined operating procedures, the temperature of the electrolyzer was increased by 0.38-2.2℃, and the cell voltage decreased by 0.23-2.32V, with a significant decrease in cell voltage.
After the implementation of electrolyzer temperature control, the total process electricity consumption decreased by 5032.23kw · h/d. Based on 350 days of operation per year, the annual savings in process electricity consumption were 1761280.5kw · h/d. The electricity fee is calculated at 0.4 RMB/(kw · h), and the investment in equipment and materials is 541,900 RMB. If depreciated over 5 years, the economic benefits is 601,551.20RMB/a.
6 conclusion
Due to differences in membrane replacement time and cell conditions, there are objective problems with deviations in cell temperature among the eight electrolyzer cells. However, due to limitations on the design of the cell temperature control process and requirements for cell temperature indicators, it is impossible to achieve optimal control over the cell temperatures of all eight cells through existing processes.At present, Inner Chlor-Alkali Plant A is taking the lead in achieving independent control of electrolyzer cell temperature within the CHLOR-ALKALI INDUSTRY. Through the implementation of this modification project, it has reduced the deviation among the temperatures of 8 electrolyzers and increased the overall operating temperature of the catholyte system, further reducing power consumption in the caustic soda process with significant energy-saving effects. It holds broad promotional significance within the industry. As an experienced engineering company in the chlor-alkali field, BPC is committed to providing upgrading services for chlor-alkali clients worldwide.

Hi! Welcome back.
How are you doing?
BPC is the leader of China for overseas chlor-alkaili marketing and sales business, as well as an engnieering company to provide chlor-alkali process and caustic soda plant service for global clients.
+86-10-67711588
Room 401-1, No. 20 Kechuang 14th Street, BDA, Beijing, China